Odisha State Board CHSE Odisha Class 11 Biology Solutions Chapter 9 Chemical Constituents of Living Cells Textbook Questions and Answers.
CHSE Odisha 11th Class Biology Chapter 9 Question Answer Chemical Constituents of Living Cells
Chemical Constituents of Living Cells Class 11 Questions and Answers CHSE Odisha
Very Short Answer Type Questions
Choose the correct option
Question 1.
The name enzyme was coined by
(a) Edward Buchner
(b) Arthuze Harden
(c) Fredrich Kunhe
(d) Names Summer
Answer:
(c) Fredrich Kunhe
Question 2.
The,first enzyme purified and crystalised.
(a) Urease
(b) Hexokinase
(c) Alcohol dehydeogenase
(d) Catalase
Answer:
(a) Urease
Question 3.
Chemically ribozyme is
(a) Protein
(b) RNA
(c) DNA
(d) Lipoprotein
Answer:
(b) RNA
Question 4.
Which of the following is known as fruit sugar?
(a) Glucose
(b) Fructose
(c) Sucrose
(d) Maltose
Answer:
(b) Fructose
Question 5.
Which of the following elements is not present in nitrogenous bases found in nucleic acids?
(a) Nitrogen
(b) Hydrogen
(c) Carbon
(d) Phosphorous
Answer:
(d) Phosphorous
Question 6.
A nucleoside is a compound of
(a) N – base + sugar + phosphate
(b) N – base + sugar
(c) N – base + phosphate
(d) sugar + phosphate
Answer:
(b) N – base + sugar
Question 7.
Maltose is a disaccharide composed of
(a) D-glucose and D-glucose
(b) D-galactose and D-glucose
(c) D-glucose and D-fructose
(d) D-glucose and L-glucose
Answer:
(a) D-glucose and D-glucose
Question 8.
Which of the following is a sulphur containing amino acids?
(a) Methionine
(b) Serine
(c) Valine
(d) Leucine
Answer:
(a) Methionine
Question 9.
Name the hydroxy amino acid from the followings.
(a) Threonine
(b) Tryptophan
(c) Phenyl alanin
(d) Valine
Answer:
(a) Threonine
Question 10.
Which one of the followings is a basic amino acid?
(a) Lysine
(b) Glycine
(c) Tryptophan
(d) Leucine
Answer:
(a) Lysine
Question 11.
Which one of the followings is an ectodermal protein?
(a) Keratin
(b) Antibodies
(c) Storage proteins
(d) Enzymes
Answer:
(a) Keratin
Fill in the blanks
Question 1.
D-glucose and L-glucose form a pair of ……………. .
Answer:
isomers
Question 2.
An aldehyde containing monosaccharide having six carbon atoms is generally called as ………….. .
Answer:
glucose
Question 3.
Inulin is found as a storage polysaccharide in the members of …………. family.
Answer:
compositae
Question 4.
…………… is known as imino acid.
Answer:
Proline
Question 5.
The derived amino acids are also known as …………. amino acids.
Answer:
non-standard
Question 6
…………… is the building block of nucleic acids.
Answer:
Nucleotide
Question 7.
The pentose sugar present in DNA is known as ……………. .
Answer:
deoxyribose
Question 8.
Butyric acid is the only fatty acids with ………….. number of carbons.
Answer:
4
Question 9.
Tocopherols are known as vitamin …………… .
Answer:
E
Question 10.
ß-carotene yields vitamin …………… .
Answer:
A
Suggest one word expression for each the following
Question 1.
The polysaccharide found as reserve food in animal.
Answer:
Glycogen
Question 2.
The compound rotating the plane of polarised light to the right.
Answer:
Dextrorotatory
Question 3.
The polysaccharide found in the exoskeleton of arthropods.
Answer:
Chitin
Question 4.
The branched strach is called as
Answer:
a-D-glucose
Question 5.
The secondary structure exhibited by silk protein.
Answer:
P-Keratin
Question 6.
A protein conformation having two or more subunits.
Answer:
Quaternary structure
Question 7.
Compounds consisting of a N-base, pentose sugar and phosphoric acid.
Answer:
Nucleotide
Question 8.
Compounds obtained by esterification of alcohol group of glycerol with fatty acids.
Answer:
Lipids
Short Answer Type Questions
Write notes on
Question 1.
Disaccharides
Answer:
(a) Disaccharides These are the sugars containing two monomeric units and can be further hydrolysed into smaller components. These are known as non-reducing sugars because the free aldehyde or ketonc group is absent, e.g., sucrose (α-D Glucose + ß- D Fructose), maltose (α-D Glucose + ß-D glucose), lactose (ß-D Galactose + α-D Glucose) etc.
Question 2.
Anomers
Answer:
Anomers These are the distereoisomers of the cyclic forms of sugars differing in the configuration at anomeric carbon 1, e.g. α-form and ß-form of glucose.
Question 3.
Homopolysaccharides
Answer:
These are those complex carbohydrates which are formed by polymerisation of only one type of monosaccharide monomers, e.g., starch, glycogen and cellulose (these all are composed of single type of monosaccharide unit namely glucose).
Question 4.
Mucopolysaccharides
Answer:
Mucopolysaccharides These are the polysaccharides forming slimy substances or mucilages and composed of mixture of simple sugars and their derivatives such as amino sugars and uranic sugars, e.g. hyaluronic acid.
Question 5.
Quaternary structure of proteins
Answer:
Quaternary Structure:
Certain proteins consist of an assembly of more than one polypeptide or subunits. These, the individual polypeptides are arranged with respect to one another (linear strings of spheres, spheres arranged one upon each other in the form of a cube or plate, etc.) e.g. haemoglobin, lactic acid dehydrogenase enzyme.
Question 6.
Non-standard amino acids
Answer:
Non-standard amino acids are the derived amino acids from the 20 standard amino acids.
e.g. hydroxyproline (derived from proline) and hydroxylysine (derived from lysine) are abundant in collagen.
Question 7.
Non-protein amino acids
Answer:
Non-protein amino acids These are natural amino acids which are not found as constituents of proteins but have some role in metabolism, e.g.
L-ornithine and L-citrulline are metabolic intermediates in urea-cycle.
ß-alanine, an isomer of alanine, occur in nature freely and a major constituent of vitamin pantothenic acid and coenzyme-A.
Question 8.
Classification of proteins based on shape
Answer:
On the basis of shape of the molecules proteins can be of two types
(i) Globular Proteins They are extensively folded and compact polypeptides having rounded shape. They are generally soluble in water and in dilute acids, alkalis, salts. They have axial ratio (length : width) of less than 10 (usually 30 H) e.g., almost all enzymes, protein hormones, blood transport proteins, antibodies (serum globulins), nutrient storage proteins, etc.
(ii) Fibrous Proteins The proteins have spiral secondary polypeptide chains wound around each other in order to form fibres. These are insoluble in water generally, but soluble in concentrated acids, alkalis and salts, e.g., collagen of connective tissue, keratin of hair, etc. They have axial ratio greater than 10, mainly of animal origin and slewes as structural or protective proteins.
Question 9.
Polyunsaturated fatty acids
Answer:
Polyunsaturated fatty acids These are several double bonds. In the most common of such acids, a methylene group separates the non-conjugated double bonds. The melting point of fatty acids decrease with increasing number of double bonds.
Question 10.
Chemical nature of enzyme
Answer:
The chemical nature of enzymes remained in dispute untils 1926, when James B Summer became succesful in purifying and crystallising the enzyme urease from jack bean. His results established that the enzyme urease is a protein. Subsequendy, John H. Northrop and Wendell M. Stanley purified and characterised a series of digestive enzymes. They confirmed Summer’s result and proved without doubt that enzymes are proteins. Since then thousands of enzymes have been purified and characterised, and all enzymes are found to be protein.
Question 11.
Activation energy
Answer:
The conversion of reactants to products in any chemical reaction is accompanied by continuous change in energy. These changes can be pictorially represented through a graph as given below. In the graph T-axis represents the potential energy content and the X-axis represents the progression of the structural transformation (states through the transition state).
Question 12.
Enzyme substrate complex.
Answer:
Each enzyme (E) has a substrate (S) binding site in its molecule in order to form a highly reactive Enzyme Substrate (ES) complex. This complex is short lived and dissociates into its product (P) and unchanged enzyme with an intermediate formation of the Enzyme Product (EP) complex.
Long Answer Type Questions
Question 1.
Describe the structure of DNA.
Answer:
Structure of DNA
The structure of DNA was elucidated by Watson and Crick based on X-ray diffraction studies. They proposed a double helix model of DNA.
According to this model, DNA exists as a double helix and consists of two strands of polynucleotides that are antiparallel to each other, i.e. both run in opposite directions, one in 5′ → 3′ direction and other in 3′ → 5′ « direction.

Diagram indicating secondary structure of DNA
The backbone of DNA is formed by the sugar phosphate-sugar chain. The nitrogen bases are projected more or less perpendicular to the backbone of DNA and faces inside. A and G of one strand base pairs with T and C, respectively on the other strand. Between A and T (A= T), there are two hydrogen bonds while, there are three hydrogen bonds between G and C (G=C).
DNA has a uniform thickness of 20 A and pitch is 34 nm. Thus, one turn of DNA measures 3.4 nm (rise per base pair) and consists of 10 nucleotides (or ten base pairs). This form of DNA is called B-DNA.
Question 2.
Describe the structures of different types of RNA.
Answer:
Ribonucleic Acid (RNA):
RNA is a polynucleotide made of ribonucleotide units having ribose sugar, phosphoric acid and one of the nitrogen bases. DNA serves as the template for the synthesis of RNA. Cellular RNAs are non-genetic and are of three types namely mRNA, /RNA and rRNA.
(i) Messenger RNA (mRNA):
It is the RNA formed during the protein synthesis. Five to ten percent of cellular RNA is of this type. The molecular weight of wRNA varies from 30000-1000000. It is short lived. DNA transfers the genetic information to ribosome through this type of RNA during the protein synthesis.
(ii) Ribosomal RNA (rRNA)
The most stable form of RNA in the cell is the rRNA. About 80% of cellular RNA is of this type. The molecular weight or rRNA ranges from 40000-1000000. It may have some folds to have a complex structure. rRNA units along with protein constitute the protein synthesising factory or the ribosome.
(iii) Transfer RNA (tRNA)
It is the smallest form of RNA made of only 75 to 100 nucleotides. It is also known as the soluble RNA. It forms about 10-15% of total cellular RNA. The molecular weight of tRNA varies from 25000-30000. It transfers the amino acids from the cytoplasm to the ribosome.
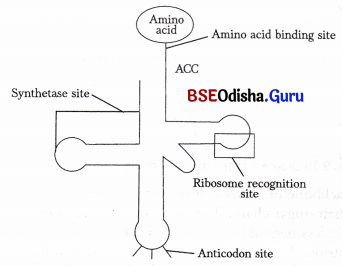
In 1964 Holley gave the detailed structure of tRNA through the ‘Clover leaf model’. In that model it was proposed that tRNA has three loops and a lump. The anticodon loop has the complementary base sequence with respect to a codon of mRNA facilitating the attachment of tRNA with the later. Other two loops are TψC loop or ribosomal binding loop and DHU loop or amino acyl synthetase binding loop. The 3’ end of rRNA ends with CCA-OH, which acts as the amino acid attachment site. The other end with G.
Question 3.
Describe the properties of enzymes indicating how they differ from chemical catalysts.
Answer:
The term ‘Enzyme’ was coined by Kuhne. Enzymes are colloidal organic macromolecules, which are mostly proteinaceous in nature except ribozyme (catalytically active RNA). These are essential for normal metabolism in the cells. Enzymes are water soluble in nature. These are useful for catalysing biochemical reactions in living cells. Hence, also known as biocatalysts.
Chemical Nature of Enzymes
The chemical nature of enzymes remained in dispute untils 1926, when James B Summer became succesful in purifying and crystallising the enzyme urease from jack bean. His results established that the enzyme urease is a protein. Subsequendy, John H. Northrop and Wendell M. Stanley purified and characterised a series of digestive enzymes.
They confirmed Summer’s result and proved without doubt that enzymes are proteins. Since then thousands of enzymes have been purified and characterised, and all enzymes are found to be protein.
As it is already studied that simple enzyme are composed of one or several polypeptide chains.
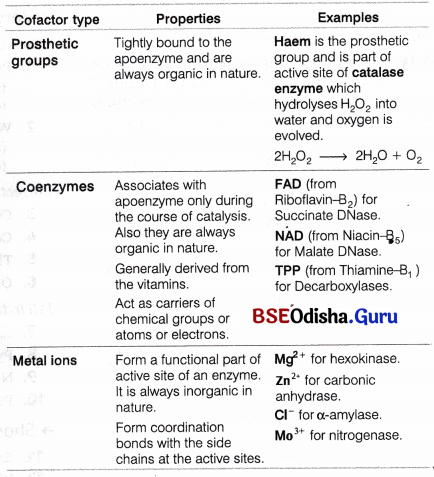
Certain enzymes possess non-protein constituents called cofactors bound to the protein part of the enzyme in order to make it catalytically active. An enzyme plus cofactor complex is called holoenzyme. An enzyme that has had its cofactor removed is called apoenzyme.
Categories of cofactor
Question 4.
Write the mechanism of enzyme actions.
Answer:
Mechanism of Enzyme Action:
The mechanism of enzyme action includes two parameters i.e. formation of enzyme, substrate complex and activation energy.
Formation of Enzyme Substrate Complex
For a chemical reaction to proceed, the substrate (S) must bind to enzyme at the active site within a cleft or pocket. During conversion of substrate into a product, formation of an enzyme-substrate complex takes place.
E (enzyme) + S (substrate) → ES complex.
This complex formation is a transient phenomenon. Thus, during the state, when substrate is bound to the enzyme active site, a new structure of the substrate, called transition state structure is formed.
After the expected reaction of breaking and making of bond is completed, the product is released from the active site and the substrate gets transformed into the structure of product.
Activation Energy:
The conversion of reactants to products in any chemical reaction is accompanied by continuous change in energy. These changes can be pictorially represented through a graph as given below. In the graph T-axis represents the potential energy content and the X-axis represents the progression of the structural transformation (states through the transition state).
Following levels are noticed between the energy levels of S and P
(i) When P is at a lower level than S, reaction is . exothermic or spontaneous, i.e. no need for external supply of energy.
(ii) When P is at higher level than S, reaction is endothermic or energy requiring reaction, i. e. external supply of energy is needed.
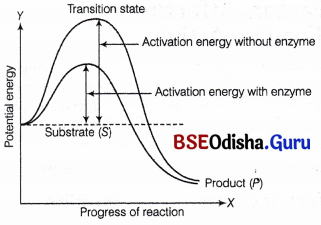
Concept of activation energy
It proves that whether it is an exothermic or an endothermic state, the ‘S ’ has to go through a much higher energy state (transition state). This difference between the average energy content of S from that of its transition state is called activation energy.
