Odisha State Board CHSE Odisha Class 12 Biology Solutions Chapter 17 Environmental Issues Textbook Questions and Answers.
CHSE Odisha 12th Class Biology Chapter 17 Question Answer Environmental Issues
Environmental Issues Class 12 Questions and Answers CHSE Odisha
Very Short Answer Type Questions
Multiple choice questions
Question 1.
Which gas leaked from Union Carbide’s Pesticide Plant in December 1984 is responsible for Bhopal Gas Tragedy?
(a) Methyl salicylate
(b) Methyl isocyanate
(c) Ammonia
(d) Hydrogen sulphide
Answer:
(b) Methyl isocyanate
Question 2.
Minamata disease is caused by the consumption of fish contaminated with
(a) lead
(b) copper
(c) zinc
(d) mercury
Answer:
(d) mercury
Question 3.
The toxic metal used as an anti-knocking agent in petrol for automobiles is
(a) chelated copper
(b) tetraethyl lead
(c) iron sulphide
(d) lead chloride
Answer:
(b) tetraethyl lead
Question 4.
Bharat Stage Emission standards came into force from the year
(a) 1998
(b) 2000
(c) 2008
(d) 2010
Answer:
(b) 2000
![]()
Question 5.
Bone and tooth decay disease is caused by drinking water contaminated with
(a) fluoride
(b) borate
(c) silicate
(d) aluminium
Answer:
(a) fluoride
Express in one or two word(s)
Question 1.
Removal of toxic substances from water by using living organisms.
Answer:
Bioremediation
Question 2.
Toxic compound formed by the reaction of carbon monoxide with haemoglobin in blood.
Answer:
Carboxyhaemoglobin
Question 3.
Enrichment of water bodies with excess amount of nutrients as a result of runoff from surrounding land leading to overgrowth of plants and algae.
Answer:
Eutrophication
Question 4.
A kind of air pollutant named for the mixture of smoke and fog in the air.
Answer:
Smog
![]()
Correct the statements, if required, by changing the underlined word (s)
Question 1.
Fifth June of each year is usually observed as Word Food security Day.
Answer:
Environment
Question 2.
The process of nutrient enrichment in water bodies is called as biomagnification.
Answer:
Eutrophication
Question 3.
Particulate matter formed by the combination of gas and water vapour is called as smog.
Answer:
Statement is correct
Question 4.
Chipko Movement was organised for the protection of water bodies.
Answer:
Forest
Fill in the blanks
Question 1.
The Environment Protection Act was enacted in
the year
Answer:
1986
Question 2.
The common refrigerant responsible for the , depletion of ozone layer of the atmosphere is ……………. .
Answer:
chlorofluorocarbon
Question 3.
Carbon monoxide binds with haemoglobin forming …………….. .
Answer:
carboxyhaemoglobin
Question 4.
Depletion of ozone layer is speeded up by the …………. atom present in CFC.
Answer:
chlorine
![]()
Short Answer Type Questions
Write within three valid points
Question 1.
Aerosol.
Answer:
- Aerosol is a colloid of fine solid particles or liquid droplets in air or another gas.
- Aerosols can be natural or anthropogenic. Examples of natural aerosols are fog and geyser steam.
Examples of artificial aerosols are haze, dust, particulate, air pollutant and smoke. - These are emitted through jet and supersonic aeroplanes. It causes depletion of ozone layer.
Question 2.
Greenhouse effect.
Answer:
Greenhouse Effect
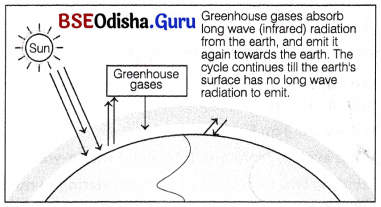
The term, ‘greenhouse effect’ has been derived from a phenomenon, which occurs inside a greenhouse. In a greenhouse, the glass panel lets the light in, but does not allow heat to escape. This results in warming up of the greenhouse.
The greenhouse effect is a naturally occurring phenomenon that is responsible for heating of earth’s surface and its atmosphere. Without greenhouse effect, the average temperature at surface of earth would have been chilly, i.e. approximately -18°C rather than the present average of 15°C.
Question 3.
Eutrophication.
Answer:
Eutrophication The process of eutrophication occurs in young lake where water is cold and clear to support life. It is the accelerated ageing of lakes due to the sewage, agricultural and industrial wastes.
The water body gets enriched with excess of nutrients such as nitrates and phosphorous promoting the overgrowth of microbes and algae. The algae release toxins in water and gradually cause deficiency of dissolved oxygen in water.
Question 4.
Acid rain.
Answer:
- The term acid rain was given by Robert August. It is rainfall with a pH of less than 5.65.
- Acid rain is a mixture of H2SO4 and HN3 and the ratio of the two may vary depending on the relative quantities of oxides of sulphur and nitrogen emitted on an average 60-70% of acidity is ascribed to H2SO4 and 30-40 % to HNO3.
- It damages foliage and growing points of plant. Causes leaching of essential minerals of soil like Ca, Mg, NO3– and SO4-2
Question 5.
Photochemical smog.
Answer:
Photochemical Smog:
Smog refers to a combination of smoke and fog formed during winter. Its formation takes place when water vapour surrounds the smoke, dust or soot particles resulting in the formation of secondary particles. These particles remain suspended in the air.
The vehicular emissions consisting of oxides of nitrogen, sulphur and hydrocarbons undergo a series of photochemical reactions forming many photochemical oxidants. This process takes place during warmer sunny days photochemical oxidants react with troposphere ozone resulting in the formation of a brownish hozy fume which is known as photochemical smog.
Question 6.
Global warming.
Answer:
Global Warming
The gradual and continuous increase in average temperature of surface of the earth has resulted in global warming.
Climate Earth temperature has increased by 0.6°C during past century, most of it in last three decades. This increased temperature caused changes in precipitation patterns.
Differentiate between the following
Question 1.
Aerosol and Smog.
Answer:
Differences between aerosol and smog are as follows
| Aerosol | Smog |
| These are present in the vapour form. Refrigerators and air conditioners use aerosol as refrigerant. | It is an opaque or dark fog having condensed water vapours, dust, smoke and gases. |
| It causes depletion of ozone layer. | It causes silvering / glazing and necrosis in plants, allergies and asthma/bronchitis in humans. |
Question 2.
Renewable resources and Non-renewable resources.
Answer:
Differences between renewable resources and non-renewable resources are as follows
| Renewable resources | Non-renewable resources |
| These resources have an ability to renew themselves in a given period of time. | These resources connot be renewed after exhaustion. |
| These are the energy resources which cannot be exhausted. | They are the energy resources which can be exhausted one day. |
| It has low carbon emission and hence environment friendly. | It causes high carbon emission and hence not environment friendly. |
| Solar energy, wind energy, tidal energy etc., are the examples of renewable resources. | Goal, petroleum, natural gases are the examples of non-renewable resources. |
![]()
Question 3.
Bioremediation and Eutrophication.
Answer:
Differences between bioremediation and eutrophication are as follows
| Bioremediation | Eutrophication |
| It is a waste management technique that involves the use of biological organisms to neutralize pollutants from a contaminated site. | It is the enrichment of a water body with nutrients. |
| Microorganisms used to perform the function of bioremediation are known as bioremediators. | This process induces growth of plants and algae and due to the biomass load may result oxygen depletion of the water body |
| It is of two types, i.e., in-situ and ex situ. | It is also of two types, i.e. accelerated and natural eutrophication. |
| It reduces pollution. | It occurs as a result of water pollution. |
Question 4.
Primary pollutants and Secondary pollutants.
Answer:
Differences between primary pollutants and secondary pollutants are as follows
| Primary pollutants | Secondary pollutants |
| These pollutants enter the environment directly from the source. | These pollutants are produced by the interaction of primary pollutants with other constituents. |
| They are less harmful. | They are more harmful. |
| They are of various categories such as particulate matter, aerosols and gases, which remain in their original form. | They generally undergo wide range of photochemical reactions and get modified. |
| e.g. CO, CO2, arsenic. | e.g. ozone, sulphuric and nitric acids. |
Long Answer Type Questions
Question 1.
Give an account of secondary air pollutants.
Answer:
Secondary Pollutants:
These are not directly emitted but are formed when primary pollutants react in atmosphere, e.g. ozone, sulphuric acid, nitric acid, Peroxyacetyl Nitrate (PAN), etc.
1. Tropospheric Ozone:
Under the influence of UV-radiation, the nitrogen dioxide released in atmosphere undergoes dissociation. This results in the formation of nitric oxide (NO) and nascent oxygen (O). This nascent oxygen undergoes reaction with molecule oxygen and forms ozone in the troposphere. Ozone traps heat causing greenhouse effect and also causes formation of photochemical smog.

Chemical reactions in the troposphere generating ozone.
2. Peroxyacetylnitrate (PAN):
The emission from vehicles contains a lot of primary pollutants.
The precursors of PAN are methyl glyoxyl, acetaldehyde and several byproducts of oxidation of aromatic compounds. The sunlight undergoes reaction with non-methane hydrocarbons and nitrogen oxides resulting in the formation of PAN. PAN is one of the important component of photochemical smog.
3. Photochemical Smog:
Smog refers to a combination of smoke and fog formed during winter. Its formation takes place when water vapour surrounds the smoke, dust or soot particles resulting in the formation of secondary particles. These particles remain suspended in the air.
The vehicular emissions consisting of oxides of nitrogen, sulphur and hydrocarbons undergo a series of photochemical reactions forming many photochemical oxidants. This process takes place during warmer sunny days photochemical oxidants react with troposphere ozone resulting in the formation of a brownish hozy fume which is known as photochemical smog. This photochemical smog causes damage to vegetation, rubber goods and irritation in eyes and lungs.
4. Acid Rain:
Primary pollutants like oxides of nitrogen, sulphur dioxide and chlorine are released in atmosphere from the fossij fuel burning, vehicular exhaust, forest fire, etc. These primary pollutants react with water vapour present in atpiosphere resulting in the formation of acids such as nitrip acid and sulphuric acid. Their acids fall on the surface of earth in the form of rain known as acid rain. The acid present in rain has harmful effects on living organisms. Acid rain causes deterioration of historical monuments. One of the examples of deterioration of monuments include Taj Mahal in Agra.
Question 2.
How can the industrial and vehicular emissions be controlled? Describe.
Answer:
Control of Industrial Emission
Industrial emission can be controlled by two practices either by confining the pollutants of gaseous nature at the source or by diluting them in the atmosphere. The first practice involves two methods.
It can be done by modifying the process of formation of pollutants so that their formation does not occur beyond the permissible level. The second method involves reducing the concentration of pollutants before they are released into the environment. These practises takes place via the following steps
(a) Combustion It is performed when the pollutants are of organic nature. It comprises of flame combustion and catalytic combustion. It is involved in the conversion of pollutants to water vapour and less harmful carbon dioxide. Catalytic combustion makes use of catalytic converters and flame combustion uses incinerator.
(b) Absorption A scrubber is used to remove or modify emitted gas. It contains a liquid absorbent through which emitted gas is passed.
(c) Adsorption In this process, the gas is passed through a porous solid adsorbent, like activated carbon silica gel and lime stone. The pollutants are held at the interface of the adsorbent.
The working of a scrubber can be described as follows Scrubber is used to remove harmful gases like SO2 from the industrial exhausts. The exhaust is passed through a spray of lime or water. Water dissolves the gases and lime reacts with SO2 to form a precipitate of calcium sulphate and sulphide.
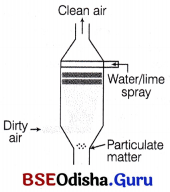
Scrubber
Control of Vehicular Emission:
Catalytic converters are fitted into automobiles (major cause of air pollution in metro cities) for reducing emissions of poisonous gases like CO and NO2. They are made with expensive metals like- platinum-palladium and rhodium as catalysts. As the exhaust passes through catalytic converter, following changes occur
(i) Unburnt hydrocarbons get burnt completely into CO2 and H2O.
(ii) Carbon monoxide and nitric oxide are converted into CO2 and N2 gases, respectively.
Motor vehicles equipped with catalytic converter should use unleaded petrol, as lead present in petrol inactivates the catalyst.
Question 3.
Write the causes of ground water pollution and state how this can be controlled.
Answer:
Ground water pollution occurs when pollutants are released to the ground and make their way down into groundwater contaminants found in groundwater cover a broad range of physical, inorganic chemical, organic chemical, bacteriological and radioactive material.
Ground water pollutants Arsenic and fluoride have been the major pollutant of ground water. The metalloid arsenic can occur naturally in groundwater. Arsenic in groundwater can also be present where mining operations or mine waste dumps that will leach arsenic. Pathogens contained in faeces can lead to ground water pollution. Viruses and protozoans commonly found in polluted groundwater. Ground water that is contaminated with pathogens can lead to fatal faecal-oral transmission of diseases.
Nitrate is the most common chemical contaminant in the world’s groundwater and aquifers. Nitrate levels above 10 mg/L in ground water can cause Blue baby syndrome.
Organic compounds are a dangerous contaminant of groundwater. They are generally introduced to the environment through careless industrial practices.
Causes of Ground Water Pollution % Natural causes The natural arsenic pollution occurs because aquifer sediments contain organic matter that generates anaerobic conditions in the aquifer. These conditions generate arsenic.
Sanitation system Ground water pollution with pathogens and nitrate can also occur from the liquids infiltrating into the ground from on site sanitation system such as pit latrines and septic tanks.
Fertilisers and pesticides Nitrate can also enter the groundwater via excessive use of fertilisers, including manure spreading. High application rates of nitrogen- containing fertilisers combined with the high water- solubility of nitrate leads to increased runoff into surface water well as leaching into groundwater.
Commercial and industrial leaks A wide variety of both inorganic and organic pollutants have been found in aquifers underlying commercial and industrial activities.
Prevention of Ground Water Pollution:
- Landfills should be properly designed, maintained and operated, located away from sensitive groundwater areas.
- Underground storage tanks should be able to meet regulatory compliance policies on their installation and maintenance.
Deep groundwater should be regularly tested and inspected.
- Fertilisers should be used in minimum amount
Question 4.
Write about the different classes of solid wastes.
Answer:
Classes of Solid Wastes:
The various classes are
1. Domestic wastes These include wastes from homes, offices, schools, etc. It generally consists of paper, leather, textile, rubber, glass, waste food materials, etc.
2. Industrial wastes These include wastes like scraps, toxic heavy metals, flyash (oxides of iron, silica and aluminium), etc., generated by industries.
3. Biomedical wastes These include disinfectants and other harmful chemicals generated by the hospitals.
4. Electronic wastes (e-wastes) comprise the damaged electronic goods and irreparable computers. It contains harmful chemicals like copper, zinc, aluminium, etc.
5. Defunct ships Old defunct ships are broken down in developing countries like India, Bangladesh and Pakistan because of cheap labour and demand for scrap metal. These ships however, possess a number of toxic materials like asbestos, lead, mercury and polychlorinated biphenyls. The people involved in ship breaking are exposed to these toxic materials and thus suffer from various diseases. The coastal areas where ship breaking is undertaken also become polluted.
6. Agricultural wastes Solid organic wastes from agricultural practice during the growing and harvesting seasons are dumped on the soil, which decompose and are washed away into nearby water bodies. These bring about eutrophication of water bodies, which affects the local biotic potential.
7. Radioactive waste Radioactive waste is generated from nuclear power plants, nuclear weapon manufacturing facilities, cancer treating hospitals and research laboratories using radioisotopes in investigations. This waste is to be disposed off safely by observing the standard guideline because if it remains for a very long period and continues to emit ionising radiation, it will be extremely hazardous to health of all forms of life.
8. Construction waste Due to demolition or construction of buildings a large amount of waste material in different forms is produced in urbon areas.
9. Extraction and processings industry waste mining and quarrying operations also produce solid wastes. Food processing industries produce a large amount of organic waste.
10. Plastic It is non-biodegradable and also increase the volume of municipal waste. Plastic has an adverse effect on animals and birds who consume it.
Burning of these results in the generation of toxic fumes which add to air pollution.
11. Waste from natural disasters Disasters like flood, earthquake, volcanic eruption and cyclone generate a lot of ash, slag, dust, smoke and organic silt.
Question 5.
What are greenhouse gases? Write about their effects on the environment.
Answer:
Greenhouse Gases
These are the gases which trap the heat causing greenhouse effect. The carbon dioxide is the most prominent one. The various greenhouse gases are
(i) Carbon Dioxide:
It is most common and abundant greenhouse gas. Its rise has been due to the large scale deforestation, change in land use and large scale combustion of fossil fuels.
Burning of petrol and diesel contributes 36%, coal contributes 35% and natural gas contributes 20% of the carbon dioxide. The carbon dioxide level has increased from 315 ppm (parts per million) in 1958 to 355 ppm in 1992 4nd then to 389 ppm in 2010. The countries which are major contributors of carbon dioxide are USA, Russia, European countries and China.
(ii) Methane
Its concentration was 700 ppb in pre-industrial times and 1750 ppb in 2000. Methane is produced by incomplete biomass combustion and incomplete decomposition mostly by anaerobic methanogens.
(iii) Nitrous Oxide
It is produced by combustion of nitrogen rich fuels, livestock wastes, breakdown of nitrogen fertilisers in soil, nitrate contaminated water, etc.
(iv) Chlorofluorocarbons(CFCs)
It is used as a common refrigerant and aerosol propellant. Bromine atom from halon used in fire extinguishers has a similar effect. These two are potent greenhouse gases.
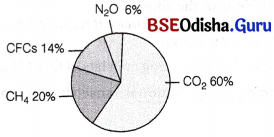
Relative contribution of various greenhouse gases to total global warming
(v) Water Vapour:
It has the capacity to trap heat radiating from the surface of the earth. Rain along with the presence of sunshine causes increase in temperature. Such climate is referred to as hot and humid.
(vi) Fluorinated Gases
These include perfluorocarbons and sulphur hexafluoride. They are industrial byproducts. They are also used as substitutes for CFCs.
(vii) Tropospheric Ozone
Nitrogen dioxide dissociates into nitric oxide and nascent oxygen in presence of UV-radiation. The nascent oxygen produced reacts with molecular oxygen forming ozone which acts as a greenhouse gas.
Question 6.
Write the causes and consequences of global warming.
Answer:
Global Warming
The gradual and continuous increase in average temperature of surface of the earth has resulted in global warming.
Effects of Global Warming:
It has been estimated by computer application studies that there may be a rise of 3°C by the year 2100 on an average.
The major effects of global warming include
1. Climate Earth temperature has increased by 0.6°C during past century, most of it in last three decades. This increased temperature caused changes in precipitation patterns.
2. Glaciers and ice caps Scientists have proposed that this rise in temperature causes deleterious changes in the environment, resulting in odd climatic changes. Thus, leading to melting of the polar ice caps and Himalayan snow caps.
3. Animals and humans The new warmer temperature conditions lead to eruption of diseases in animals and thousands of species will become extinct in a very short period of time. People from coastal areas will start migrating due to climate change.
4. Ocean and coasts The increase in temperature causes melting of polar ice caps and glaciers. This will result in the rise of ocean water level. The increased level of ocean water will cause the submerging of many island nations and coastal cities. The high temperature will cause accelerated vanishing of coral reefs.
5. Water and agriculture The increased temperature will cause decreased productivity in agricultural practice.
Reducing Greenhouse Gases
Due to the harmful effets of global warming, there was a need to control it. For this following steps were taken
1. World Meterological Organisation and United Nations Environment Programme (UNEP) jointly set up the Intergovernmental Panel on Climate Change (IPCC) in 1988.
2. At Earth Summit (1992), there was created the United Nations Framework Convention on Climate Change (UNFCC) which came into force in 1994.
3. Kyoto Protocol (1997) was signed by 160 countries to reduce the emission of CO 2 NO, CH4 by 5% and also to reduce CFCs emission.
4. Copenhagen conference was held in 2009 under UNFCC. However, it failed as there was no unanimity in agreement among the participating countries.
